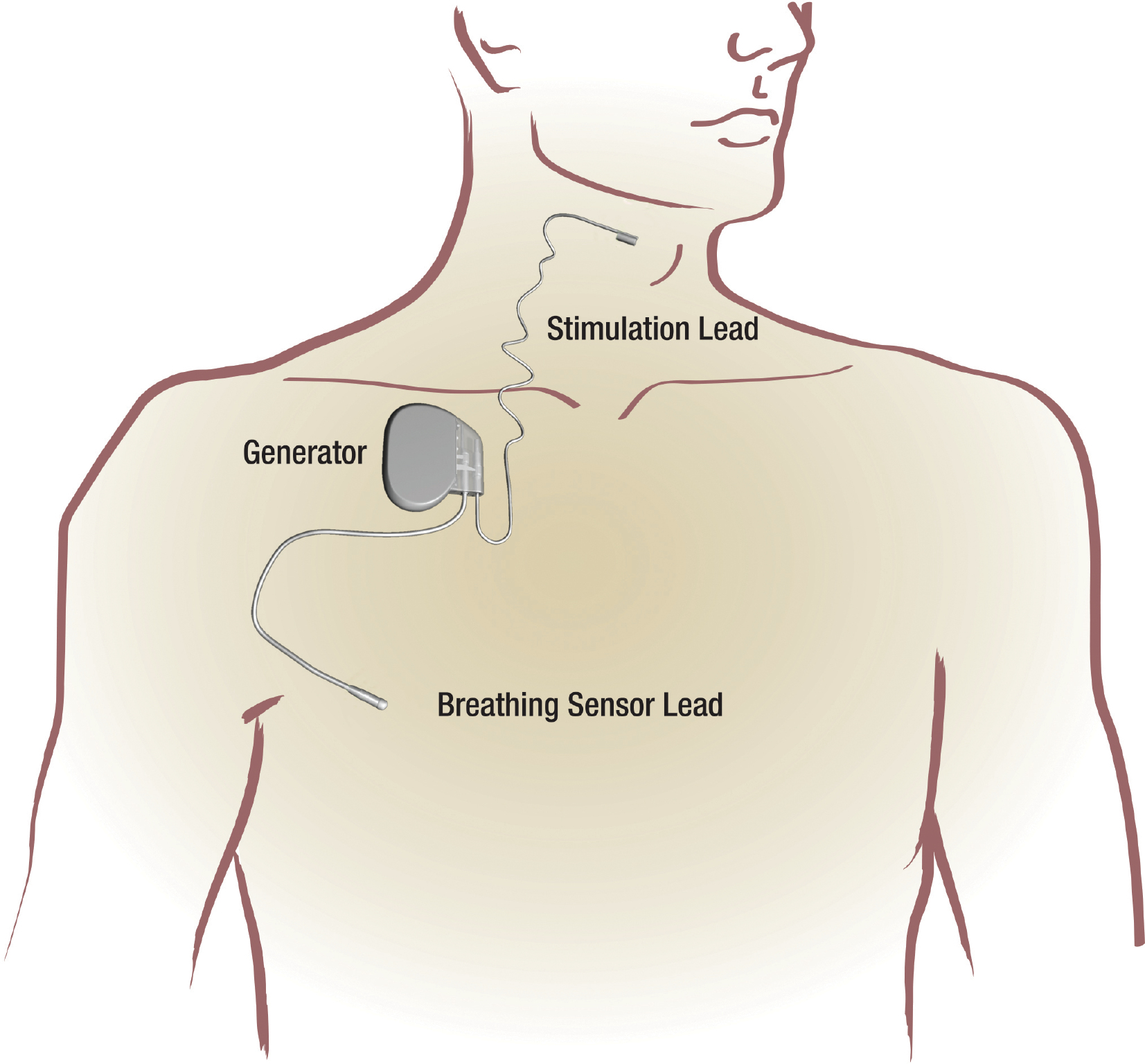
Inspire Device For Sleep Apnea Mri Safety . 20 rows inspire mri guidelines for inspire therapy: patients who require mri should discuss inspire therapy with their doctor. Announced that the us fda has approved additional mri scan conditions for use. As with any surgically implanted device, there are. the united states food and drug administration (fda) has approved additional magnetic resonance imaging (mri) scan. Inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients. Please see the “mri guidelines for inspire implant” manual. For details, refer to the “mri guidelines for inspire uas therapy”. conditional device are able to undergo an mri scan. patients with inspire model 3028 can undergo mri if certain conditions and precautions are met. inspire medical systems, inc.
from naenta.com
conditional device are able to undergo an mri scan. inspire medical systems, inc. Announced that the us fda has approved additional mri scan conditions for use. 20 rows inspire mri guidelines for inspire therapy: Inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients. Please see the “mri guidelines for inspire implant” manual. As with any surgically implanted device, there are. patients who require mri should discuss inspire therapy with their doctor. the united states food and drug administration (fda) has approved additional magnetic resonance imaging (mri) scan. patients with inspire model 3028 can undergo mri if certain conditions and precautions are met.
Learn More About Inspire Sleep Therapy North Atlanta ENT & Allergy
Inspire Device For Sleep Apnea Mri Safety Announced that the us fda has approved additional mri scan conditions for use. Please see the “mri guidelines for inspire implant” manual. patients who require mri should discuss inspire therapy with their doctor. Announced that the us fda has approved additional mri scan conditions for use. conditional device are able to undergo an mri scan. 20 rows inspire mri guidelines for inspire therapy: the united states food and drug administration (fda) has approved additional magnetic resonance imaging (mri) scan. As with any surgically implanted device, there are. Inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients. For details, refer to the “mri guidelines for inspire uas therapy”. patients with inspire model 3028 can undergo mri if certain conditions and precautions are met. inspire medical systems, inc.
From radiopaedia.org
Inspire device (radiographs) Image Inspire Device For Sleep Apnea Mri Safety Please see the “mri guidelines for inspire implant” manual. the united states food and drug administration (fda) has approved additional magnetic resonance imaging (mri) scan. As with any surgically implanted device, there are. For details, refer to the “mri guidelines for inspire uas therapy”. patients who require mri should discuss inspire therapy with their doctor. 20 rows. Inspire Device For Sleep Apnea Mri Safety.
From www.tmc.edu
A device to help veterans breathe easier TMC News Inspire Device For Sleep Apnea Mri Safety patients who require mri should discuss inspire therapy with their doctor. patients with inspire model 3028 can undergo mri if certain conditions and precautions are met. Announced that the us fda has approved additional mri scan conditions for use. As with any surgically implanted device, there are. Please see the “mri guidelines for inspire implant” manual. Inspire therapy. Inspire Device For Sleep Apnea Mri Safety.
From uthscent.com
Success Story Inspire Device for Sleep Apnea & Snoring UTHSC ENT Inspire Device For Sleep Apnea Mri Safety Please see the “mri guidelines for inspire implant” manual. Announced that the us fda has approved additional mri scan conditions for use. conditional device are able to undergo an mri scan. inspire medical systems, inc. Inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients. patients who require mri should discuss inspire therapy. Inspire Device For Sleep Apnea Mri Safety.
From sleepreviewmag.com
FDA Approves Implanted Device for Sleep Apnea Sleep Review Inspire Device For Sleep Apnea Mri Safety the united states food and drug administration (fda) has approved additional magnetic resonance imaging (mri) scan. patients who require mri should discuss inspire therapy with their doctor. conditional device are able to undergo an mri scan. Please see the “mri guidelines for inspire implant” manual. 20 rows inspire mri guidelines for inspire therapy: patients with. Inspire Device For Sleep Apnea Mri Safety.
From youarecurrent.com
Inspire treats sleep apnea with implanted device and remote control Inspire Device For Sleep Apnea Mri Safety Inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients. patients with inspire model 3028 can undergo mri if certain conditions and precautions are met. As with any surgically implanted device, there are. For details, refer to the “mri guidelines for inspire uas therapy”. Announced that the us fda has approved additional mri scan conditions. Inspire Device For Sleep Apnea Mri Safety.
From sleepreviewmag.com
FDA Grants Inspire AHI and BMI Increase Sleep Review Inspire Device For Sleep Apnea Mri Safety patients who require mri should discuss inspire therapy with their doctor. Inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients. As with any surgically implanted device, there are. conditional device are able to undergo an mri scan. the united states food and drug administration (fda) has approved additional magnetic resonance imaging (mri). Inspire Device For Sleep Apnea Mri Safety.
From cybersectors.com
Inspire sleep apnea reviews {June 2021} All You Need To Know! Cyber Inspire Device For Sleep Apnea Mri Safety patients who require mri should discuss inspire therapy with their doctor. Please see the “mri guidelines for inspire implant” manual. inspire medical systems, inc. Announced that the us fda has approved additional mri scan conditions for use. 20 rows inspire mri guidelines for inspire therapy: the united states food and drug administration (fda) has approved additional. Inspire Device For Sleep Apnea Mri Safety.
From www.slhduluth.com
New Technology for Treating Sleep Apnea St. Luke's Ear, Nose and Inspire Device For Sleep Apnea Mri Safety Announced that the us fda has approved additional mri scan conditions for use. As with any surgically implanted device, there are. For details, refer to the “mri guidelines for inspire uas therapy”. patients who require mri should discuss inspire therapy with their doctor. Please see the “mri guidelines for inspire implant” manual. patients with inspire model 3028 can. Inspire Device For Sleep Apnea Mri Safety.
From sleepreviewmag.com
Inspire Surpasses 20,000 Patient Milestone for Its Implanted Inspire Device For Sleep Apnea Mri Safety the united states food and drug administration (fda) has approved additional magnetic resonance imaging (mri) scan. For details, refer to the “mri guidelines for inspire uas therapy”. Inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients. 20 rows inspire mri guidelines for inspire therapy: Please see the “mri guidelines for inspire implant” manual.. Inspire Device For Sleep Apnea Mri Safety.
From gambolnews.com
Understanding Inspire Sleep Apnea and 4 Discovering Remedies Inspire Device For Sleep Apnea Mri Safety Announced that the us fda has approved additional mri scan conditions for use. Please see the “mri guidelines for inspire implant” manual. patients who require mri should discuss inspire therapy with their doctor. Inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients. 20 rows inspire mri guidelines for inspire therapy: conditional device. Inspire Device For Sleep Apnea Mri Safety.
From naenta.com
Learn More About Inspire Sleep Therapy North Atlanta ENT & Allergy Inspire Device For Sleep Apnea Mri Safety the united states food and drug administration (fda) has approved additional magnetic resonance imaging (mri) scan. patients who require mri should discuss inspire therapy with their doctor. inspire medical systems, inc. Inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients. For details, refer to the “mri guidelines for inspire uas therapy”. . Inspire Device For Sleep Apnea Mri Safety.
From katu.com
Implanted sleep apnea device promises safer, uninterrupted sleep Inspire Device For Sleep Apnea Mri Safety 20 rows inspire mri guidelines for inspire therapy: patients with inspire model 3028 can undergo mri if certain conditions and precautions are met. Inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients. inspire medical systems, inc. Please see the “mri guidelines for inspire implant” manual. For details, refer to the “mri guidelines. Inspire Device For Sleep Apnea Mri Safety.
From stopsnoringguide.xyz
Inspire Sleep Apnea Implant Review The Stop Snoring Guide Inspire Device For Sleep Apnea Mri Safety 20 rows inspire mri guidelines for inspire therapy: inspire medical systems, inc. patients with inspire model 3028 can undergo mri if certain conditions and precautions are met. For details, refer to the “mri guidelines for inspire uas therapy”. the united states food and drug administration (fda) has approved additional magnetic resonance imaging (mri) scan. conditional. Inspire Device For Sleep Apnea Mri Safety.
From www.fairfaxent.com
Inspire ™ Sleep Apnea Therapy Inspire Device For Sleep Apnea Mri Safety Inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients. inspire medical systems, inc. For details, refer to the “mri guidelines for inspire uas therapy”. 20 rows inspire mri guidelines for inspire therapy: Please see the “mri guidelines for inspire implant” manual. the united states food and drug administration (fda) has approved additional. Inspire Device For Sleep Apnea Mri Safety.
From www.youtube.com
Inspire Device designed to stop sleep apnea and improve your sleep Inspire Device For Sleep Apnea Mri Safety Announced that the us fda has approved additional mri scan conditions for use. inspire medical systems, inc. patients with inspire model 3028 can undergo mri if certain conditions and precautions are met. Please see the “mri guidelines for inspire implant” manual. Inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients. patients who. Inspire Device For Sleep Apnea Mri Safety.
From www.nbcdfw.com
How the Inspire device works to treat sleep apnea NBC 5 DallasFort Worth Inspire Device For Sleep Apnea Mri Safety inspire medical systems, inc. patients with inspire model 3028 can undergo mri if certain conditions and precautions are met. the united states food and drug administration (fda) has approved additional magnetic resonance imaging (mri) scan. conditional device are able to undergo an mri scan. Inspire therapy is indicated for moderate to severe obstructive sleep apnea in. Inspire Device For Sleep Apnea Mri Safety.
From www.youtube.com
Inspire sleep apnea device YouTube Inspire Device For Sleep Apnea Mri Safety As with any surgically implanted device, there are. 20 rows inspire mri guidelines for inspire therapy: conditional device are able to undergo an mri scan. the united states food and drug administration (fda) has approved additional magnetic resonance imaging (mri) scan. For details, refer to the “mri guidelines for inspire uas therapy”. Please see the “mri guidelines. Inspire Device For Sleep Apnea Mri Safety.
From www.slhduluth.com
New Technology for Treating Sleep Apnea St. Luke's Ear, Nose and Inspire Device For Sleep Apnea Mri Safety Please see the “mri guidelines for inspire implant” manual. patients who require mri should discuss inspire therapy with their doctor. Inspire therapy is indicated for moderate to severe obstructive sleep apnea in adult patients. the united states food and drug administration (fda) has approved additional magnetic resonance imaging (mri) scan. conditional device are able to undergo an. Inspire Device For Sleep Apnea Mri Safety.